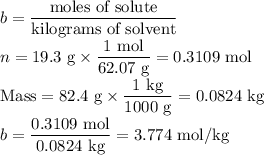
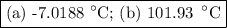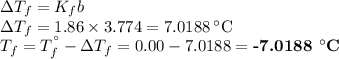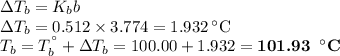An ethylene glycol solution contains 19.3g of ethylene glycol (c2h6o2) in 82.4ml of water.
(a)...

Chemistry, 01.08.2019 05:10 pricillagarcia2002
An ethylene glycol solution contains 19.3g of ethylene glycol (c2h6o2) in 82.4ml of water.
(a) compute the freezing point of the solution. (assume a density of 1.00 g/ml for water.)
(b)compute the boiling point of the solution. (assume a density of 1.00 g/ml for water.)
express your answer using five significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Strong conductivity of plasma allows it to act and react as and
Answers: 2

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3

Chemistry, 23.06.2019 01:00
How does carbon monoxide pose the greatest threat to humans? a. it can be produced by wood fires. b. it can be produced by home furnaces. c. it is produced by acid rain. d. it is produced by modern automobiles.
Answers: 2
You know the right answer?
Questions



Mathematics, 06.10.2021 14:00




Mathematics, 06.10.2021 14:00

Biology, 06.10.2021 14:00


Physics, 06.10.2021 14:00

Mathematics, 06.10.2021 14:00

Business, 06.10.2021 14:00

Mathematics, 06.10.2021 14:00



Mathematics, 06.10.2021 14:00

Mathematics, 06.10.2021 14:00

Mathematics, 06.10.2021 14:00


Mathematics, 06.10.2021 14:00