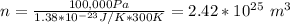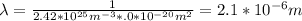Chemistry, 01.08.2019 04:30 ddatsman1730
What is the mean free path for the molecules in an ideal gas when the pressure is 100 kpa and the temperature is 300 k given that the collision cross-section for the molecules of that gas is 2.0 × 10-20 m2? boltzmann's constant is k = 1.38 × 10-23 j/k.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 23.06.2019 01:30
In what way do investigations build scientific knowledge? the results of investigations lead to questions that cannot be tested. they reflect the opinions and social values of scientists, ensuring valid information. the results of investigations lead to new questions, which lead to new investigations. they are not influenced by the research of earlier scientists, so they are able to address gaps in understanding.i
Answers: 1
You know the right answer?
What is the mean free path for the molecules in an ideal gas when the pressure is 100 kpa and the te...
Questions

Mathematics, 16.10.2020 04:01

Medicine, 16.10.2020 04:01

Biology, 16.10.2020 04:01

Mathematics, 16.10.2020 04:01


Mathematics, 16.10.2020 04:01

Mathematics, 16.10.2020 04:01




Social Studies, 16.10.2020 04:01





History, 16.10.2020 04:01

Mathematics, 16.10.2020 04:01


Mathematics, 16.10.2020 04:01