
Chemistry, 01.08.2019 04:10 ramirezdolores
Determine the rate law and the value of k for the following reaction using the data provided. co(g) cl2(g) → cocl2(g)[co]i (m)[cl2]i(m)initial rate (m-1s-1)0.250.400.6960.250.801.970. 500.803.94a) rate

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which one of the following gases is not an important component of soil?
Answers: 2

Chemistry, 21.06.2019 17:40
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1
You know the right answer?
Determine the rate law and the value of k for the following reaction using the data provided. co(g)...
Questions

Mathematics, 19.05.2021 23:20

Mathematics, 19.05.2021 23:20

Mathematics, 19.05.2021 23:20


Mathematics, 19.05.2021 23:20


English, 19.05.2021 23:20

English, 19.05.2021 23:20

Mathematics, 19.05.2021 23:20

Mathematics, 19.05.2021 23:20

Mathematics, 19.05.2021 23:20


Chemistry, 19.05.2021 23:20




Mathematics, 19.05.2021 23:20

Mathematics, 19.05.2021 23:20


Social Studies, 19.05.2021 23:20

![\text{Rate}=k[CO]^1[Cl_2]^{\frac{3}{2}}](/tpl/images/0156/8683/d3cd0.png) and value of 'k' is
and value of 'k' is 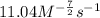
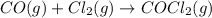
![\text{Rate}=k[CO]^a[Cl_2]^b](/tpl/images/0156/8683/d760d.png)
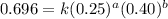 ....(1)
....(1)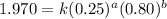 ....(2)
....(2)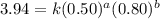 ....(3)
....(3)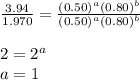
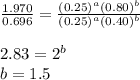
![0.696=k[0.25]^1[0.40]^{\frac{3}{2}}\\\\k=11.04M^{-\frac{7}{2}}s^{-1}](/tpl/images/0156/8683/569d9.png)



