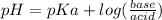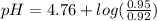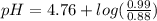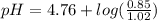Calculate the ph of 1.00 l of the buffer 0.95 m ch3coona/0.92 m ch3cooh before and after the addition of the following species. (assume there is no change in volume.) (a) ph of starting buffer: (b) ph after addition of 0.040 mol naoh: (c) ph after further addition of 0.100 mol hcl:

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1
You know the right answer?
Calculate the ph of 1.00 l of the buffer 0.95 m ch3coona/0.92 m ch3cooh before and after the additio...
Questions

English, 28.07.2019 04:00



Spanish, 28.07.2019 04:00



Mathematics, 28.07.2019 04:00



Chemistry, 28.07.2019 04:00







History, 28.07.2019 04:00

History, 28.07.2019 04:00

Social Studies, 28.07.2019 04:00