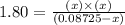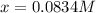Chemistry, 01.08.2019 02:20 tinapersaud1587
Phosphorus pentachloride decomposes according to the chemical equation pcl5(g)↽−−⇀pcl3(g)+cl2(=1.80 at 250 ∘c a 0.349 mol sample of pcl5(g) is injected into an empty 4.00 l reaction vessel held at 250 ∘c. calculate the concentrations of pcl5(g) and pcl3(g) at equilibrium.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2

Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3
You know the right answer?
Phosphorus pentachloride decomposes according to the chemical equation pcl5(g)↽−−⇀pcl3(g)+cl2(=1.80...
Questions

English, 30.07.2021 14:00

Business, 30.07.2021 14:00

Biology, 30.07.2021 14:00



Computers and Technology, 30.07.2021 14:00

English, 30.07.2021 14:00

Mathematics, 30.07.2021 14:00

Social Studies, 30.07.2021 14:00

Health, 30.07.2021 14:00


Business, 30.07.2021 14:00

Geography, 30.07.2021 14:00


Mathematics, 30.07.2021 14:00





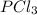 and
and 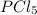 at equilibrium are, 0.0834 M and 0.00385 M
at equilibrium are, 0.0834 M and 0.00385 M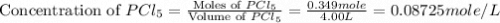
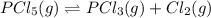
![K_c=\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0156/5529/73fe0.png)