
Chemistry, 01.08.2019 00:10 morris9878
The equilibrium constant for the dissociation of acetic acid at 25oc is 1.85x10-5. what will be the equilibrium concentration of h+ at 25oc, if the initial concentration of acetic acid was 0.100 m? ch3cooh (aq) ↔ ch3coo- (aq) + h+ (aq) give your answer to two significant figures and in decimal form.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1
You know the right answer?
The equilibrium constant for the dissociation of acetic acid at 25oc is 1.85x10-5. what will be the...
Questions

Chemistry, 03.12.2021 04:10

Chemistry, 03.12.2021 04:10

Mathematics, 03.12.2021 04:10

Chemistry, 03.12.2021 04:10

Mathematics, 03.12.2021 04:10






History, 03.12.2021 04:10




Mathematics, 03.12.2021 04:10

Business, 03.12.2021 04:10


English, 03.12.2021 04:10

Mathematics, 03.12.2021 04:10

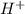 is
is 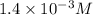
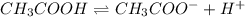
![\frac{[CH_{3}COO^{-}][H^{+}]}{[CH_{3}COOH]}](/tpl/images/0156/2235/23a00.png) =
= 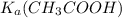
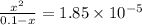
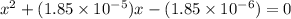
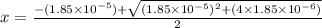 M
M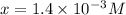
![[H^{+}]](/tpl/images/0156/2235/85507.png) =x=
=x=

