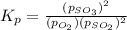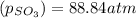Chemistry, 31.07.2019 23:10 yournerdybirdyp43oi3
At 900.0 k, the equilibrium constant (kp) for the following reaction is 0.345. 2so2 + o2 (g) →2 so3 (g) at equilibrium, the partial pressure of so2 is 36.9 atm and that of o2 is 16.8 atm. the partial pressure of so3 is atm.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3



Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1
You know the right answer?
At 900.0 k, the equilibrium constant (kp) for the following reaction is 0.345. 2so2 + o2 (g) →2 so3...
Questions

Mathematics, 22.02.2021 22:40

History, 22.02.2021 22:40

Social Studies, 22.02.2021 22:40

Mathematics, 22.02.2021 22:40

Mathematics, 22.02.2021 22:40

Mathematics, 22.02.2021 22:40

Mathematics, 22.02.2021 22:40

Mathematics, 22.02.2021 22:40


Social Studies, 22.02.2021 22:40



Mathematics, 22.02.2021 22:40


Biology, 22.02.2021 22:40





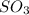 is, 88.84 atm
is, 88.84 atm
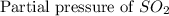 = 36.9 atm
= 36.9 atm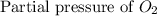 = 16.8 atm
= 16.8 atm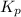 will be,
will be,