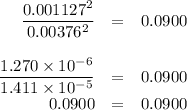Chemistry, 31.07.2019 22:20 Laners0219
The value of kc for the reaction between water vapor and dichlorine monoxide, h2o(g) 1 cl2o(g) 4 2 hocl(g) is 0.0900 at 25°c. determine the equilibrium concentrations of all three compounds at 25°c if the starting concentrations of both reactants are 0.00432 m and no hocl is present.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2
You know the right answer?
The value of kc for the reaction between water vapor and dichlorine monoxide, h2o(g) 1 cl2o(g) 4 2 h...
Questions

Mathematics, 21.01.2022 14:00




Mathematics, 21.01.2022 14:00


Biology, 21.01.2022 14:00

Mathematics, 21.01.2022 14:00




Mathematics, 21.01.2022 14:00

Mathematics, 21.01.2022 14:00

Mathematics, 21.01.2022 14:00

History, 21.01.2022 14:00





Mathematics, 21.01.2022 14:00

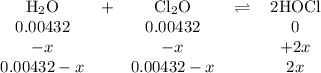
![K_{\text{c}} = \dfrac{\text{[HOCl]$^{2}$}}{\text{[H$_{2}$O][Cl$_2$O]}} = \dfrac{(2x)^{2}}{(0.00432 - x)^{2}} = 0.0900\\\\\begin{array}{rcl}\dfrac{4x^{2}}{(0.00432 - x)^{2}} &=& 0.0900\\ \dfrac{2x }{0.00432 - x} & = & 0.300\\2x & = & 0.300(0.00432 - x)\\2x & = & 0.001296 - 0.300x\\2.300x & = & 0.001296\\x & = & \mathbf{5.63\times 10^{-4}}\\\end{array}](/tpl/images/0155/9177/30633.png)