
Chemistry, 31.07.2019 21:20 smelcher3900
Hydrogen peroxide decomposes into water and oxygen in a first-order process. h2o2(aq) --> h2o(l) + 1/2 o2(g)at 20.0 °c, the half-life for the reaction is 3.92 x 104 seconds. if the initial concentration of hydrogen peroxide is 0.52 m, what is the concentration after 7.00 days? 1.2 x 10-5 m0.034 m0.074 m0.22 m0.52 m

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:10
Imagine that you have produced several versions of lactase, each of which differs from normal lactase by a single amino acid. describe a test that could indirectly determine which of the versions significantly alters the three-dimensional shape of the lactase protein.
Answers: 2

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1
You know the right answer?
Hydrogen peroxide decomposes into water and oxygen in a first-order process. h2o2(aq) --> h2o(l)...
Questions

Mathematics, 05.12.2020 18:00

Mathematics, 05.12.2020 18:00

Mathematics, 05.12.2020 18:00


Chemistry, 05.12.2020 18:00

Mathematics, 05.12.2020 18:00

Mathematics, 05.12.2020 18:00



Mathematics, 05.12.2020 18:00

Mathematics, 05.12.2020 18:00

Mathematics, 05.12.2020 18:00


Social Studies, 05.12.2020 18:00



Geography, 05.12.2020 18:00

Mathematics, 05.12.2020 18:00


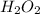 after 7.00 days is
after 7.00 days is 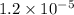 M
M![[H_{2}O_{2}]=[H_{2}O_{2}]_{0}\times (0.5)^{(\frac{t}{t_{0.5}})}](/tpl/images/0155/7555/57b7f.png)
![[H_{2}O_{2}]_{0}](/tpl/images/0155/7555/0e2b6.png) is initial concentration of
is initial concentration of 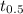 is half-life
is half-life![[H_{2}O_{2}]= 0.52\times (0.5)^{\frac{604800}{39200}}](/tpl/images/0155/7555/78886.png)
![[H_{2}O_{2}]](/tpl/images/0155/7555/955b6.png) =
= 

