
The decomposition of hydrogen peroxide follows first order kinetics and has a rate constant of 2.54 x 10-4 s-1 at a certain temperature. if the concentration of hydrogen peroxide is 0.321 m after 855 s , what was the initial concentration of hydrogen peroxide at this temperature? 0.258 m0.399 m0.538 m0.677 m1.48 m

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 02:30
24 points and brainliest to anyone who can answer under 10 minutes with best ! the table below shows the role of different substances during photosynthesis. substance role during photosynthesis glucose stores chemical energy water combines with glucose to form carbon dioxide chlorophyll traps sunlight which of the following statements would correct one of the roles listed in the table? glucose combines with carbon to form water. chlorophyll reacts with light to produce carbon dioxide. water combines with carbon dioxide during photosynthesis. chlorophyll stores chemical energy needed for photosynthesis.
Answers: 1

Chemistry, 22.06.2019 19:00
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2
You know the right answer?
The decomposition of hydrogen peroxide follows first order kinetics and has a rate constant of 2.54...
Questions

English, 25.01.2021 23:20

Computers and Technology, 25.01.2021 23:20

Chemistry, 25.01.2021 23:20

English, 25.01.2021 23:20

History, 25.01.2021 23:20


Mathematics, 25.01.2021 23:20

Chemistry, 25.01.2021 23:20






English, 25.01.2021 23:20

Mathematics, 25.01.2021 23:20





![k=\frac{2.303}{t}\log\frac{[A_o]}{[A]}](/tpl/images/0155/7548/f1041.png)
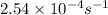
![[A_o]](/tpl/images/0155/7548/dc622.png) = initial amount of the reactant = ?
= initial amount of the reactant = ?![2.54\times 10^{-4}s^{-1}=\frac{2.303}{855s}\log \frac{[A_o]}{0.321}](/tpl/images/0155/7548/e899e.png)
![[A_o]=0.399M](/tpl/images/0155/7548/ee654.png)




