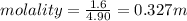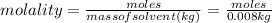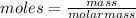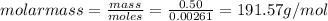Chemistry, 31.07.2019 20:40 linshweyioo5442
Aforensic chemist is given a white powder for analysis. she dissolves 0.50 g of the substance in 8.0 g of benzene. the solution freezes at 3.9°c. can the chemist conclude that the compound is cocaine (c17h21n04)? what assumptions are made in the analysis? the freezing point of benzene is 5.5°c.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1


You know the right answer?
Aforensic chemist is given a white powder for analysis. she dissolves 0.50 g of the substance in 8.0...
Questions




History, 04.02.2020 18:45

Mathematics, 04.02.2020 18:45

Social Studies, 04.02.2020 18:45

Physics, 04.02.2020 18:45


World Languages, 04.02.2020 18:45

History, 04.02.2020 18:45

Mathematics, 04.02.2020 18:45

Mathematics, 04.02.2020 18:45

Mathematics, 04.02.2020 18:45

History, 04.02.2020 18:45

Mathematics, 04.02.2020 18:45


Biology, 04.02.2020 18:45



Health, 04.02.2020 18:45