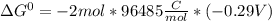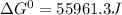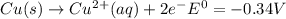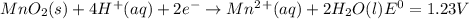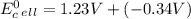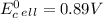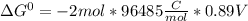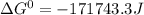Use tabulated electrode potentials to calculate ∆g° rxn for each reaction at 25 °c in kj.
(a)...


Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1

Chemistry, 23.06.2019 04:00
What two categories of toxins were present in the air at dish,texas as a result of the gas pipelines that pass through the area
Answers: 1

You know the right answer?
Questions

Mathematics, 14.12.2020 02:40

Mathematics, 14.12.2020 02:40





French, 14.12.2020 02:40


Health, 14.12.2020 02:40

Mathematics, 14.12.2020 02:40

Mathematics, 14.12.2020 02:40

Mathematics, 14.12.2020 02:40

English, 14.12.2020 02:40

Biology, 14.12.2020 02:40


English, 14.12.2020 02:40

Mathematics, 14.12.2020 02:40


Mathematics, 14.12.2020 02:40

Mathematics, 14.12.2020 02:40

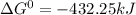 , (b)
, (b) 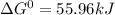 and (c)
and (c) 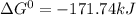
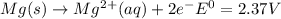
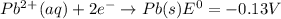
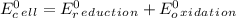
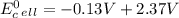
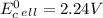
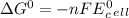
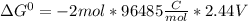
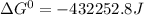
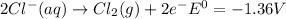
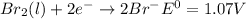
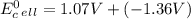
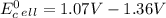

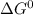 same as we did for part a.
same as we did for part a.