
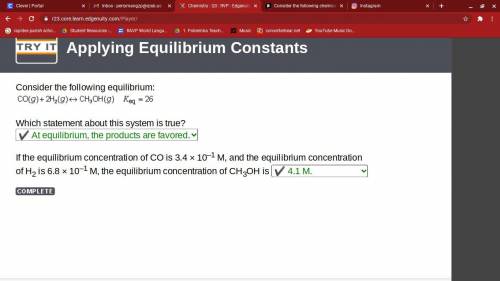
Consider the following chemical reaction: co (g) + 2h2(g) ↔ ch3oh(g) at equilibrium in a particular experiment, the concentrations of co and h2 were 0.15 m and0.36 m, respectively. what is the equilibrium concentration of ch3oh? the value of keq for this reaction is 14.5 at the temperature of the experiment. what is the equilibrium concentration of ch3oh?
a) 14.5b) 7.61 x 10 ^ -3c) 2.82 x 10 ^ -1d) 3.72 x 10 ^ -3e) 1.34 x 10 ^ -3

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

You know the right answer?
Consider the following chemical reaction: co (g) + 2h2(g) ↔ ch3oh(g) at equilibrium in a particular...
Questions

Mathematics, 13.12.2020 04:20

Advanced Placement (AP), 13.12.2020 04:20





SAT, 13.12.2020 04:20

Mathematics, 13.12.2020 04:20

Chemistry, 13.12.2020 04:20


Health, 13.12.2020 04:20

Physics, 13.12.2020 04:20


Mathematics, 13.12.2020 04:20




Mathematics, 13.12.2020 04:20

Mathematics, 13.12.2020 04:20

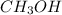 will be, (C)
will be, (C) 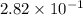
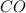 at equilibrium = 0.15 M
at equilibrium = 0.15 M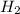 at equilibrium = 0.36 M
at equilibrium = 0.36 M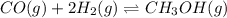
![K_c=\frac{[CH_3OH]}{[CO][H_2]^2}](/tpl/images/0155/4298/4cf94.png)
![14.5=\frac{[CH_3OH]}{(0.15)\times (0.36)^2}](/tpl/images/0155/4298/2d443.png)
![[CH_3OH]=2.82\times 10^{-1}M](/tpl/images/0155/4298/5b3a4.png)



