
Chemistry, 31.07.2019 19:10 brianlykid3042
Calculate the percent ionization of formic acid (hco2h) in a solution that is 0.125 m in formic acid. the ka of formic acid is 1.77 ⋅ 10-4. calculate the percent ionization of formic acid (hco2h) in a solution that is 0.125 m in formic acid. the ka of formic acid is 1.77 10-4. 0.859 0.0180 3.79 2.25 ⋅ 10-5 6.94

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 23.06.2019 00:00
How many peaks will be present in a mass spectrum for brcl?
Answers: 1
You know the right answer?
Calculate the percent ionization of formic acid (hco2h) in a solution that is 0.125 m in formic acid...
Questions

English, 28.08.2019 11:00

Chemistry, 28.08.2019 11:00


History, 28.08.2019 11:00



Mathematics, 28.08.2019 11:00

Mathematics, 28.08.2019 11:00

Mathematics, 28.08.2019 11:00




History, 28.08.2019 11:00

History, 28.08.2019 11:00



Mathematics, 28.08.2019 11:00

Mathematics, 28.08.2019 11:00


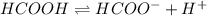
![\frac{[HCOO^{-}][H^{+}]}{[HCOOH]}=K_{a}(HCOOCH)](/tpl/images/0155/4252/45db7.png)
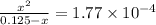

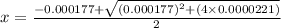
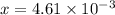 M
M![[H^{+}]=4.61\times 10^{-3}M](/tpl/images/0155/4252/38c16.png)
![\frac{[H^{+}]}{initial concentration of HCOOH}\times 100](/tpl/images/0155/4252/17262.png) =
= 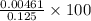 = 3.69%
= 3.69%

