Chemistry, 31.07.2019 18:30 bixbylily95
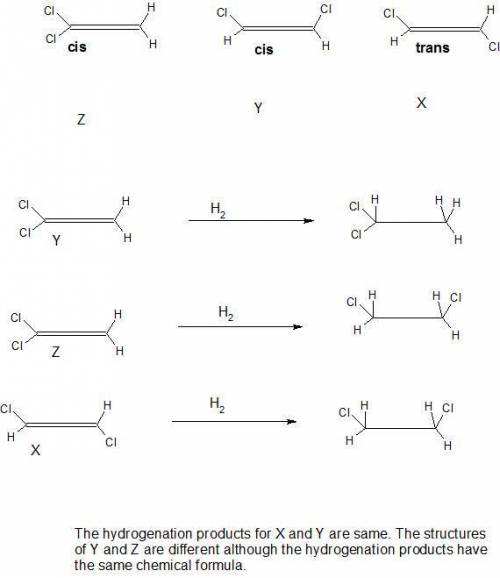
Be sure to answer all parts. there are three different dichloroethylenes (molecular formula c2h2cl2), which we can designate x, y, and z. compound x has no dipole moment, but compound z does. compounds x and z each combine with hydrogen to give the same product: c2h2cl2(x or z) + h2 → clch2―ch2cl what are the structures of x, y, and z? be sure to include lone pair electrons

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 23.06.2019 00:10
Find the missing probability in the table below a.0.10 b.40 c.0.80 d. 0.20
Answers: 2

Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1
You know the right answer?
Be sure to answer all parts. there are three different dichloroethylenes (molecular formula c2h2cl2)...
Questions





Mathematics, 12.05.2021 05:50


Mathematics, 12.05.2021 05:50

Arts, 12.05.2021 05:50



History, 12.05.2021 05:50




Mathematics, 12.05.2021 05:50


Social Studies, 12.05.2021 05:50

History, 12.05.2021 05:50

Social Studies, 12.05.2021 05:50

Mathematics, 12.05.2021 05:50