

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 01:30
Ascientist conducted an experiment and discovered that certain plants grow faster when given a particular amount of fertilizer. anouther scientist conducted the same experiment and got similar results. which concept does this best illustrate? a) repetition b) replication c) precision d) validity
Answers: 2

Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2

Chemistry, 23.06.2019 15:50
Many radioactive atoms that have large masses undergo radioactive decay by releasing a particle that is identical to a helium-4 nucleus. what changes in the original atom are expected as a result of this natural phenomenon? the atomic number and the mass number will decrease. the atomic number and the mass number will increase. the atomic number will increase, and the mass number will decrease. the atomic number will decrease, and the mass number will increase.
Answers: 2

Chemistry, 23.06.2019 17:30
Hydrogen-2 is also known as deuterium as well as hydrogen-3 is known as tritium hydrogen-1 is our common hydrogen isotope a sample hydrogen gas has 99% hydrogen -1 ,0.8% deuterium , and 0.2% tritium what is the average atomic mass of this mixture of isotope to the thousands place
Answers: 1
You know the right answer?
Which process is expected to have an increase in entropy?
a. formation of liquid water from...
a. formation of liquid water from...
Questions

Mathematics, 02.10.2020 19:01

Chemistry, 02.10.2020 19:01


Mathematics, 02.10.2020 19:01

Mathematics, 02.10.2020 19:01

Mathematics, 02.10.2020 19:01



History, 02.10.2020 19:01


Biology, 02.10.2020 19:01




Mathematics, 02.10.2020 19:01



Mathematics, 02.10.2020 19:01

Biology, 02.10.2020 19:01

Chemistry, 02.10.2020 19:01

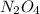 decomposes then the reaction will be as follows.
decomposes then the reaction will be as follows.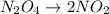
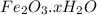 .
.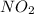 gas is the process that is expected to have an increase in entropy.
gas is the process that is expected to have an increase in entropy.

