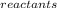Calculate the entropy change for the reaction: fe2o3(s) +3c(s) -> 2fe(s) + 3co(g)
entropy...

Chemistry, 30.07.2019 23:40 villafana36
Calculate the entropy change for the reaction: fe2o3(s) +3c(s) -> 2fe(s) + 3co(g)
entropy data:
fe2o3(s): 90 j/k mol
c(s): 5.7 j/k mol
fe(s): 27.2 j/k mol
co(g): 198 j/k mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3

Chemistry, 23.06.2019 01:30
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2
You know the right answer?
Questions


Mathematics, 18.11.2020 20:50

Mathematics, 18.11.2020 20:50

Social Studies, 18.11.2020 20:50




Mathematics, 18.11.2020 20:50

Mathematics, 18.11.2020 20:50



Mathematics, 18.11.2020 20:50


Mathematics, 18.11.2020 20:50


Chemistry, 18.11.2020 20:50


Arts, 18.11.2020 20:50


 - ∑S
- ∑S