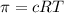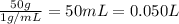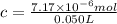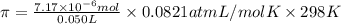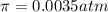Chemistry, 30.07.2019 23:30 makwoods417ow2txa
Lysozyme extracted from chicken egg white has a molar mass of 13,930 g mo1-1. exactly 0.1 g of this protein is dissolved in 50 g of water at 298 k. calculate the vapor pressure lowering, the depression in freezing point, the elevation of boiling point, and the osmotic pressure of this solution. the vapor pressure of pure water at 298 k is 23. 76 mmhg.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 23.06.2019 03:00
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3

Chemistry, 23.06.2019 04:31
How many grams of iron can be made from 16.5 grams of fe2o3
Answers: 1

Chemistry, 23.06.2019 09:30
The earth's surface is (science) a: studied using seismic waves b: constantly changing over time c: only studied indirectly d: the same today as million of years
Answers: 1
You know the right answer?
Lysozyme extracted from chicken egg white has a molar mass of 13,930 g mo1-1. exactly 0.1 g of this...
Questions

Mathematics, 13.10.2020 15:01

Mathematics, 13.10.2020 15:01

Biology, 13.10.2020 15:01


Mathematics, 13.10.2020 15:01

Spanish, 13.10.2020 15:01



Mathematics, 13.10.2020 15:01


Mathematics, 13.10.2020 15:01

Mathematics, 13.10.2020 15:01

Mathematics, 13.10.2020 15:01



Engineering, 13.10.2020 15:01




Social Studies, 13.10.2020 15:01

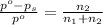
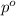 = Vapor Pressure of the pure solvent
= Vapor Pressure of the pure solvent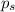 = Vapor Pressure of the solution
= Vapor Pressure of the solution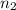 moles of solute
moles of solute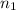 = moles of solvent
= moles of solvent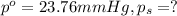
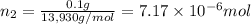
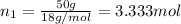
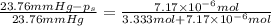
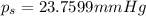
 is given by:
is given by: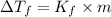
 = molal depression constant of solvent
= molal depression constant of solvent
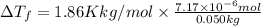
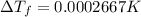
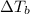 is given by:
is given by: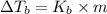
 = molal elevation constant of solvent
= molal elevation constant of solvent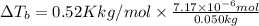
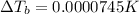
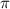 is given as:
is given as: