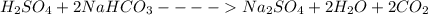Chemistry, 30.07.2019 04:20 Diegosolorzano50
Sodium bicarbonate is sometimes used to neutralize acid spills. if 2.0 l of 1.0 m h2so4 is spilled, what mass of sodium bicarbonate (nahco3) is required to neutralize all the h2so4?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1

Chemistry, 23.06.2019 11:30
Distilled water is a completely neutral solution. what is its ph? a. 1 b. 7 c. 14 d. 0
Answers: 2

Chemistry, 23.06.2019 12:10
Which structure is a valid representation of a hydrocarbon molecule?
Answers: 2
You know the right answer?
Sodium bicarbonate is sometimes used to neutralize acid spills. if 2.0 l of 1.0 m h2so4 is spilled,...
Questions


Mathematics, 07.07.2019 20:00

History, 07.07.2019 20:00


Chemistry, 07.07.2019 20:00



History, 07.07.2019 20:00




Computers and Technology, 07.07.2019 20:00


Health, 07.07.2019 20:00

Mathematics, 07.07.2019 20:00

Computers and Technology, 07.07.2019 20:00


Biology, 07.07.2019 20:00