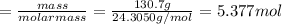Chemistry, 30.07.2019 04:10 luvpeaceandsocc6312
Assuming an efficiency of 49.50%, calculate the actual yield of magnesium nitrate formed from 130.7 g of magnesium and excess copper(ii) nitrate. mg+cu(no3)2⟶mg(no3)2+cu actual yield:

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1

Chemistry, 23.06.2019 00:00
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1

Chemistry, 23.06.2019 02:10
Detrimental the length of the object shown 1. 97.8mm 2. 97.80 mm 3. 97mm 4. 98mm
Answers: 2
You know the right answer?
Assuming an efficiency of 49.50%, calculate the actual yield of magnesium nitrate formed from 130.7...
Questions



English, 26.02.2020 23:50

Mathematics, 26.02.2020 23:50

Mathematics, 26.02.2020 23:50



Mathematics, 26.02.2020 23:50

Computers and Technology, 26.02.2020 23:50





Mathematics, 26.02.2020 23:50

History, 26.02.2020 23:50

Computers and Technology, 26.02.2020 23:50


Business, 26.02.2020 23:50