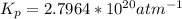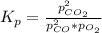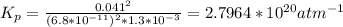Chemistry, 30.07.2019 03:20 nengliangli523
Determine the value of kp for the following reaction if the equilibrium concentrations are as follows: p(co)eq = 6.8 × 10-11 atm, p(o2)eq = 1.3 × 10-3 atm, p(co2)eq = 0.041 atm. 2 co(g) + o2(g) ⇌ 2 co2(g)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2


Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1
You know the right answer?
Determine the value of kp for the following reaction if the equilibrium concentrations are as follow...
Questions




Mathematics, 10.03.2020 03:34

Mathematics, 10.03.2020 03:34


Mathematics, 10.03.2020 03:34

History, 10.03.2020 03:34


Mathematics, 10.03.2020 03:34


Mathematics, 10.03.2020 03:34