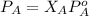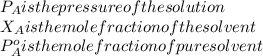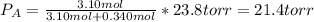Chemistry, 30.07.2019 02:10 keviongardner
If 0.340 mol of a nonvolatile nonelectrolyte are dissolved in 3.10 mol of water, what is the vapor pressure ph2o of the resulting solution? the vapor pressure of pure water is 23.8 torr at 25 ∘c . express your answer with the appropriate units.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2

Chemistry, 23.06.2019 03:10
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2
You know the right answer?
If 0.340 mol of a nonvolatile nonelectrolyte are dissolved in 3.10 mol of water, what is the vapor p...
Questions

Social Studies, 22.01.2020 15:31

English, 22.01.2020 15:31


English, 22.01.2020 15:31


Mathematics, 22.01.2020 15:31


Biology, 22.01.2020 15:31


Mathematics, 22.01.2020 15:31



Mathematics, 22.01.2020 15:31


Social Studies, 22.01.2020 15:31