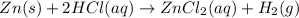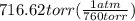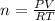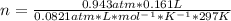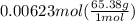Chemistry, 30.07.2019 02:10 jamarengle2
If 161 ml of wet h2 is collected over water at 24 ∘c and a barometric pressure of 739 torr, how many grams of zn have been consumed? (the vapor pressure of water is 22.38 torr.) m m = .420 g

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1

You know the right answer?
If 161 ml of wet h2 is collected over water at 24 ∘c and a barometric pressure of 739 torr, how many...
Questions



Mathematics, 29.01.2021 02:50

History, 29.01.2021 02:50




History, 29.01.2021 02:50

Mathematics, 29.01.2021 02:50


Mathematics, 29.01.2021 02:50


Computers and Technology, 29.01.2021 02:50

Mathematics, 29.01.2021 02:50


Mathematics, 29.01.2021 02:50

Chemistry, 29.01.2021 02:50