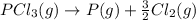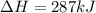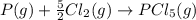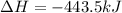Chemistry, 30.07.2019 01:20 jones03riley
Given the enthalpies of reaction: 2p(g) + 3cl2(g) → 2pcl3(g) dh = –574 kj 2p(g) + 5cl2(g) → 2pcl5(g) dh = –887 kj what is the enthalpy change of the following reaction: pcl3(g) + cl2(g) → pcl5(g

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which of the following statements is true about planck’s law
Answers: 1

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2


Chemistry, 23.06.2019 03:30
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1
You know the right answer?
Given the enthalpies of reaction: 2p(g) + 3cl2(g) → 2pcl3(g) dh = –574 kj 2p(g) + 5cl2(g) → 2pcl5(g...
Questions


History, 26.11.2019 22:31

Mathematics, 26.11.2019 22:31

Mathematics, 26.11.2019 22:31


Mathematics, 26.11.2019 22:31

Mathematics, 26.11.2019 22:31

History, 26.11.2019 22:31


Mathematics, 26.11.2019 22:31


History, 26.11.2019 22:31

Social Studies, 26.11.2019 22:31

Mathematics, 26.11.2019 22:31

Mathematics, 26.11.2019 22:31



Health, 26.11.2019 22:31

Chemistry, 26.11.2019 22:31

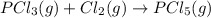
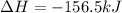
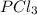 on reactant side. When we reversed an equation then the sign of enthalpy change is also changed.
on reactant side. When we reversed an equation then the sign of enthalpy change is also changed.