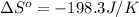Chemistry, 30.07.2019 01:10 nockturnal1993
Calculate the entropy change for the surroundings of the reaction below at 350k: n2(g) + 3h2(g) -> 2nh3(g) entropy data: nh3 = 192.5 j/mol k h2 = 130.6 j/mol k n2 = 191.5 j/mol k

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1

Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1
You know the right answer?
Calculate the entropy change for the surroundings of the reaction below at 350k: n2(g) + 3h2(g) -&g...
Questions


Spanish, 31.08.2020 23:01



Mathematics, 31.08.2020 23:01


Mathematics, 31.08.2020 23:01



Mathematics, 31.08.2020 23:01


Mathematics, 31.08.2020 23:01

French, 31.08.2020 23:01

History, 31.08.2020 23:01

Mathematics, 31.08.2020 23:01

Mathematics, 31.08.2020 23:01



Computers and Technology, 31.08.2020 23:01

Chemistry, 31.08.2020 23:01

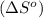 .
.
![\Delta S^o=[n_{NH_3}\times \Delta S^0_{(NH_3)}]-[n_{N_2}\times \Delta S^0_{(N_2)}+n_{H_2}\times \Delta S^0_{(H_2)}]](/tpl/images/0148/7107/be4ea.png)
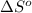 = entropy of reaction = ?
= entropy of reaction = ?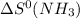 = standard entropy of
= standard entropy of 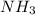
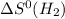 = standard entropy of
= standard entropy of 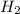
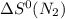 = standard entropy of
= standard entropy of 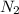
![\Delta S^o=[2mole\times (192.5J/K.mole)]-[1mole\times (191.5J/K.mole)+3mole\times (130.6J/K.mole)]](/tpl/images/0148/7107/cd252.png)