Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 02:00
Why does ammonia, nh3, behave as a base when it reacts with an acid? z
Answers: 2


Chemistry, 23.06.2019 07:30
Which statement explains which thermometer is more appropriate to measure the temperature of a liquid at 43.6 degrees celsius a) thermometer a, because it measures temperature more accurately than thermometer b b) thermometer b, because it measures temperature more accurately than thermometer a c) thermometer a, because it measures temperature more precisely than thermometer b d) thermometer b, because it measures temperature more precisely than thermometer a
Answers: 2

Chemistry, 23.06.2019 07:30
Assignment directions: pick one of the following chemists and perform a bit of research on him/her. answer the following questions. alice hamilton rosalind franklin marie curie gertrude b. elion ada yonath henry cavendish robert boyle antoine lavoisier mario j. molina svante arrhenius
Answers: 1
You know the right answer?
Acompound weighing 0.458 g is dissolved in 30.0 g of acetic acid. the freezing point of the solution...
Questions

Mathematics, 23.09.2020 20:01





English, 23.09.2020 20:01


History, 23.09.2020 20:01









Mathematics, 23.09.2020 20:01

Mathematics, 23.09.2020 20:01

English, 23.09.2020 20:01

Chemistry, 23.09.2020 20:01

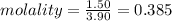
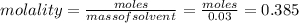
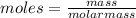
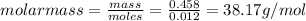
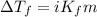
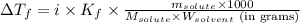
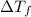 = Depression in freezing point = 1.50 K = 1.50°C (Change remains constant)
= Depression in freezing point = 1.50 K = 1.50°C (Change remains constant)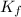 = molal freezing point elevation constant = 3.90°C/m
= molal freezing point elevation constant = 3.90°C/m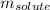 = Given mass of solute = 0.458 g
= Given mass of solute = 0.458 g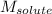 = Molar mass of solute (glucose) = ? g/mol
= Molar mass of solute (glucose) = ? g/mol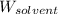 = Mass of solvent (acetic acid) = 30.0 g
= Mass of solvent (acetic acid) = 30.0 g