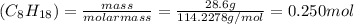Chemistry, 29.07.2019 23:30 Gladistshiala267
Consider the combustion reaction for octane (c8h18), which is a primary component of gasoline. 2c8h18+25o2⟶16co2+18h2o how many moles of co2 are emitted into the atmosphere when 28.6 g c8h18 is burned?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2
You know the right answer?
Consider the combustion reaction for octane (c8h18), which is a primary component of gasoline. 2c8h1...
Questions

Computers and Technology, 17.02.2021 09:20

Biology, 17.02.2021 09:20

Mathematics, 17.02.2021 09:20


English, 17.02.2021 09:20


English, 17.02.2021 09:20

English, 17.02.2021 09:20


Mathematics, 17.02.2021 09:20

Computers and Technology, 17.02.2021 09:20

Chemistry, 17.02.2021 09:20

Mathematics, 17.02.2021 09:20

Social Studies, 17.02.2021 09:20


Computers and Technology, 17.02.2021 09:20



Mathematics, 17.02.2021 09:30

Mathematics, 17.02.2021 09:30