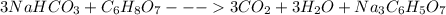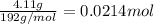Antacids, such as alka-seltzer, use the reaction of sodium bicarbonate with citric acid in water solution to produce a fizz as follows: 3nahco3 + c6h8o7 → 3co2 + 3h2o + na3c6h5o7 if 4.11 g of the citric acid (c6h8o7, mw = 192 g/mol) react with excess sodium bicarbonate (nahco3), how many grams of carbon dioxide (co2, mw = 44 g/mol) are formed as the solution fizzes?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1

Chemistry, 23.06.2019 02:40
How can a mixture of salt water be separated into salt and water
Answers: 1

Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3
You know the right answer?
Antacids, such as alka-seltzer, use the reaction of sodium bicarbonate with citric acid in water sol...
Questions

Health, 01.08.2019 03:30

Mathematics, 01.08.2019 03:30


Mathematics, 01.08.2019 03:30

Mathematics, 01.08.2019 03:30




Geography, 01.08.2019 03:30

Social Studies, 01.08.2019 03:30

Mathematics, 01.08.2019 03:30

English, 01.08.2019 03:30

Health, 01.08.2019 03:30

English, 01.08.2019 03:30

Mathematics, 01.08.2019 03:30

Mathematics, 01.08.2019 03:30


Social Studies, 01.08.2019 03:30