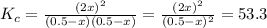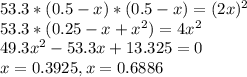Chemistry, 29.07.2019 21:10 sreeranjanig
At a certain temperature, the equilibrium constant, , for this reaction is 53.3. h2(g)+i2(g)↽−−⇀2hi(=53.3 at this temperature, 0.500 mol h2 and 0.500 mol i2 were placed in a 1.00 l container to react. what concentration of hi is present at equilibrium?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 23.06.2019 07:00
Which of the following statements is true? an atom consists of protons, electrons, and neutrons.an atom consists of protons and neutrons.an atom consists of electrons bonded to one another.an atom consists of protons bonded to one another.
Answers: 1

Chemistry, 23.06.2019 10:30
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 3.75 mol fe and 8.70 mol nio(oh) react?
Answers: 1
You know the right answer?
At a certain temperature, the equilibrium constant, , for this reaction is 53.3. h2(g)+i2(g)↽−−⇀2hi(...
Questions

Social Studies, 08.03.2021 05:00


Chemistry, 08.03.2021 05:00


Mathematics, 08.03.2021 05:00

Mathematics, 08.03.2021 05:00


Mathematics, 08.03.2021 05:00

Social Studies, 08.03.2021 05:00

Physics, 08.03.2021 05:00








Social Studies, 08.03.2021 05:00


Social Studies, 08.03.2021 05:00

![K_{c}=\frac{[HI]^{2}}{[I_{2}][H_{2}]}](/tpl/images/0148/0680/ff3a2.png)
![[HI]={[HI]}_{0}+2x\\{[H_{2}]}={[H_{2}]}_{0}-x\\{[I_{2}]}={[I_{2}]}_{0}-x\\](/tpl/images/0148/0680/e5b4e.png)