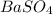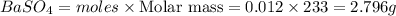Chemistry, 29.07.2019 19:10 crystalclear99
How many grams of barium sulfate can be produced from the reaction of 2.54 grams sodium sulfate and 2.54 g barium chloride? na2so4(aq) + bacl2(aq) --> baso4(s) + 2nacl(aq) report your answer to 3 decimal places.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1


Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2
You know the right answer?
How many grams of barium sulfate can be produced from the reaction of 2.54 grams sodium sulfate and...
Questions



Mathematics, 16.11.2019 22:31



Computers and Technology, 16.11.2019 22:31

Mathematics, 16.11.2019 22:31

Mathematics, 16.11.2019 22:31

Business, 16.11.2019 22:31




English, 16.11.2019 22:31

History, 16.11.2019 22:31



Mathematics, 16.11.2019 22:31



English, 16.11.2019 22:31

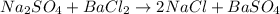
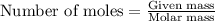
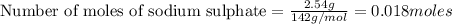
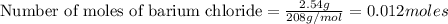
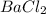 reacts with 1 mole of
reacts with 1 mole of 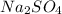
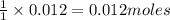 of
of