

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
You know the right answer?
The enthalpy change for converting 10.0 g of ice at -25.0°c to water at 80.0°c is kj. the specific...
Questions




Biology, 31.05.2021 17:00

Mathematics, 31.05.2021 17:00

Social Studies, 31.05.2021 17:00

Mathematics, 31.05.2021 17:00

Computers and Technology, 31.05.2021 17:00




Mathematics, 31.05.2021 17:00




English, 31.05.2021 17:00

History, 31.05.2021 17:00

English, 31.05.2021 17:00

Computers and Technology, 31.05.2021 17:00

Mathematics, 31.05.2021 17:00

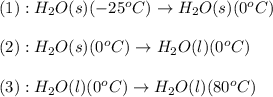
![\Delta H=[m\times c_{p,s}\times (T_{final}-T_{initial})]+n\times \Delta H_{fusion}+[m\times c_{p,l}\times (T_{final}-T_{initial})]](/tpl/images/0137/4660/5cd06.png)
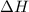 = enthalpy change = ?
= enthalpy change = ?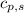 = specific heat of solid water = 2.09 J/gk
= specific heat of solid water = 2.09 J/gk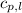 = specific heat of liquid water = 4.18 J/gk
= specific heat of liquid water = 4.18 J/gk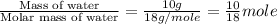
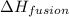 = enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole
= enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole![\Delta H=[10g\times 2.09J/gK\times (273-248)k]+\frac{10}{18}mole\times 6010J/mole+[10g\times 4.18J/gK\times (353-273)k]](/tpl/images/0137/4660/7e566.png)
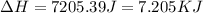 (1 KJ = 1000 J)
(1 KJ = 1000 J)

