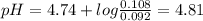Chemistry, 27.07.2019 03:20 carolinasoto
Abuffer is prepared by mixing 204.0 ml of 0.452 mol/l hydrochloric acid (hci) and 500.0 ml of 0.400 mol/l sodium acetate (nachco2, naac). what is the ph of the above buffer solution?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3

Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1
You know the right answer?
Abuffer is prepared by mixing 204.0 ml of 0.452 mol/l hydrochloric acid (hci) and 500.0 ml of 0.400...
Questions



English, 14.08.2021 07:00

Mathematics, 14.08.2021 07:00

History, 14.08.2021 07:00



Geography, 14.08.2021 07:00

Mathematics, 14.08.2021 07:00


Business, 14.08.2021 07:00

Biology, 14.08.2021 07:00



Chemistry, 14.08.2021 07:00


Chemistry, 14.08.2021 07:00

Advanced Placement (AP), 14.08.2021 07:00


Physics, 14.08.2021 07:00

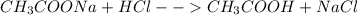
![\frac{[salt]}{[acid]}](/tpl/images/0137/3111/a0d10.png)