
Chemistry, 26.07.2019 20:30 joeblaszak4776
Three kilograms of steam is contained in a horizontal, frictionless piston and the cylinder is heated at a constant pressure of 0.5 bar from 100 °c to such a temperature that the specific volume increases by 2.5 times. if the amount of heat that must be added to accomplish this change is 500 kj, calculate the final temperature of the steam, the expansion work, and the change in internal energy.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1
You know the right answer?
Three kilograms of steam is contained in a horizontal, frictionless piston and the cylinder is heate...
Questions







Mathematics, 19.02.2021 23:30





Mathematics, 19.02.2021 23:30



Mathematics, 19.02.2021 23:30


Chemistry, 19.02.2021 23:30

Spanish, 19.02.2021 23:30


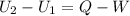
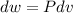
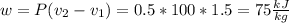 (There is a multiplication by 100 due to the conversion of bar to kPa)
(There is a multiplication by 100 due to the conversion of bar to kPa)
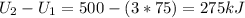
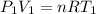
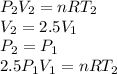
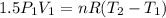
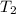 :
: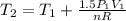
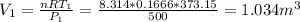
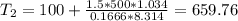 ºC
ºC


