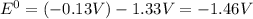Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Asample of radium-226 will decay 1/4 of its original amount after 3200years. what is the half-life of radium-226?
Answers: 2

Chemistry, 22.06.2019 01:30
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1


Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
Use tabulated standard electrode potentials to calculate the standard cell potential for the followi...
Questions





Mathematics, 28.12.2020 21:30



Mathematics, 28.12.2020 21:30



Mathematics, 28.12.2020 21:30



English, 28.12.2020 21:30


World Languages, 28.12.2020 21:30


English, 28.12.2020 21:30

Law, 28.12.2020 21:30

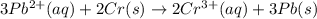
![E^0_{[Pb^{2+}/Pb]}=-0.13V](/tpl/images/0133/7776/82211.png)
![E^0_{[Cr^{3+}/Cr]}=1.33V](/tpl/images/0133/7776/e4046.png)
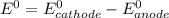
![E^0=E^0_{[Pb^{2+}/Pb]}-E^0_{[Cr^{3+}/Cr]}](/tpl/images/0133/7776/e8ef0.png)