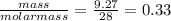Chemistry, 26.07.2019 04:20 tgreenberg2002
Magnesium and nitrogen react in a combination reaction to produce magnesium nitride: 3 mg + n2 → mg3n2 in a particular experiment, a 9.27-g sample of n2 reacts completely. the mass of mg consumed is g.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1
You know the right answer?
Magnesium and nitrogen react in a combination reaction to produce magnesium nitride: 3 mg + n2 → mg...
Questions




Mathematics, 02.11.2020 06:10

Mathematics, 02.11.2020 06:10



Mathematics, 02.11.2020 06:10

Mathematics, 02.11.2020 06:10





SAT, 02.11.2020 06:10

Mathematics, 02.11.2020 06:10


Mathematics, 02.11.2020 06:10

Mathematics, 02.11.2020 06:10

Mathematics, 02.11.2020 06:10