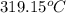Chemistry, 26.07.2019 04:10 hairyears3394
What celsius temperature, t2, is required to change the volume of the gas sample in part a (t1 = 23 ∘c , v1= 1.69×103 l ) to a volume of 3.38×103 l ? assume no change in pressure or the amount of gas in the balloon.

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 11:00
Find the enthalpy of neutralization of hcl and naoh. 87 cm3 of 1.6 mol dm-3 hydrochloric acid was neutralized by 87 cm3 of 1.6 mol dm-3 naoh. the temperature rose from 298 k to 317.4 k. the specific heat capacity is the same as water, 4.18 j/k g. a. -101.37 kj b. 7055 kj c. 10,1365 kj
Answers: 1

Chemistry, 23.06.2019 13:30
The two isotopes of chlorine are 3517cl and 3717cl. which isotope is the most abundant?
Answers: 1

Chemistry, 23.06.2019 14:00
What can happen to an atoms electrons when an electric current is passed through the atom?
Answers: 1

Chemistry, 23.06.2019 20:00
Within nonmetals,ions that have more electrons tend to be bigger than ions that have fewer additional electrons why?
Answers: 1
You know the right answer?
What celsius temperature, t2, is required to change the volume of the gas sample in part a (t1 = 23...
Questions





Biology, 21.05.2020 07:04

Biology, 21.05.2020 07:04


Mathematics, 21.05.2020 07:04


History, 21.05.2020 07:57





History, 21.05.2020 07:57



Biology, 21.05.2020 07:57

Mathematics, 21.05.2020 07:57

![\frac{T_{2} }{V_{2} } = \frac{T_{1} }{V_{1} } \\Which can be rewritten as T_{2} = \frac{T_{1} V_{2} }{V_{1} }if you make T2 the subject of the formula. This formula is true only if temperature is in Kelvin not degrees Celsius so T1 must be converted to KelvinNow to calculate T2[tex]T_{2}= \frac{296.15K*3.38.10^{3}L }{1.69.10^{3}L }= 592.3K](/tpl/images/0133/6170/71b6f.png) =
=