
In the first step of the ostwald process for the synthesis of nitric acid, ammonia is converted to nitric oxide by the high-temperature reaction 4 nh31g2 + 5 o21g2 ¡ 4 no1g2 + 6 h2o1g2 (a) how is the rate of consumption of o2 related to the rate of consumption of nh3? (b) how are the rates of formation of no and h2o related to the rate of consumption of nh3

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which term describes a fracture in the earth at which land stays in the same place? a. joint b. fault c. split d. hinge
Answers: 1


Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

You know the right answer?
In the first step of the ostwald process for the synthesis of nitric acid, ammonia is converted to n...
Questions


English, 16.10.2020 20:01


English, 16.10.2020 20:01


Mathematics, 16.10.2020 20:01

Mathematics, 16.10.2020 20:01


History, 16.10.2020 20:01




Mathematics, 16.10.2020 20:01

Mathematics, 16.10.2020 20:01


Biology, 16.10.2020 20:01

Geography, 16.10.2020 20:01



Mathematics, 16.10.2020 20:01

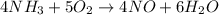
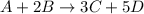
![rate=-\frac{\Delta [A]}{\Delta t}=-\frac{1}{2}\frac{\Delta [B]}{\Delta t}=\frac{1}{3}\frac{\Delta [C]}{\Delta t}=\frac{1}{5}\frac{\Delta [D]}{\Delta t}](/tpl/images/0133/4682/3644a.png)
![rate=-\frac{1}{4}\frac{\Delta [NH_3]}{\Delta t}=-\frac{1}{5}\frac{\Delta [O_2]}{\Delta t}=\frac{1}{4}\frac{\Delta [NO]}{\Delta t}=\frac{1}{6}\frac{\Delta [H_2O]}{\Delta t}](/tpl/images/0133/4682/51a20.png)
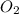 related to rate of consumption of
related to rate of consumption of 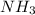 as:
as:![-\frac{1}{5}\frac{\Delta [O_2]}{\Delta t}=-\frac{1}{4}\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/1a857.png)
![\frac{\Delta [O_2]}{\Delta t}=\frac{5}{4}\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/ce2db.png)
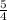 the rate of consumption of ammonia.
the rate of consumption of ammonia.![\frac{1}{4}\frac{\Delta [NO]}{\Delta t}=-\frac{1}{4}\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/91a55.png)
![\frac{\Delta [NO]}{\Delta t}=-\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/c5068.png)
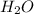 to the rate of consumption of ammonia would be:
to the rate of consumption of ammonia would be:![\frac{1}{6}\frac{\Delta [H_2O]}{\Delta t}=-\frac{1}{4}\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/41d66.png)
![\frac{\Delta [H_2O]}{\Delta t}=-\frac{6}{4}\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/66644.png)
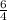 times that is 1.5 times to the rate of consumption of ammonia.
times that is 1.5 times to the rate of consumption of ammonia. 

