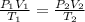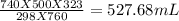Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1
You know the right answer?
Asample of nitrogen gas had a volume of 500. ml, a pressure in its closed container of 740 torr, and...
Questions

Geography, 21.08.2019 22:30


Chemistry, 21.08.2019 22:30

Mathematics, 21.08.2019 22:30

History, 21.08.2019 22:30

Social Studies, 21.08.2019 22:30

Advanced Placement (AP), 21.08.2019 22:30

History, 21.08.2019 22:30


Mathematics, 21.08.2019 22:30

Biology, 21.08.2019 22:30


Social Studies, 21.08.2019 22:30

History, 21.08.2019 22:30



History, 21.08.2019 22:30

Mathematics, 21.08.2019 22:30


History, 21.08.2019 22:30