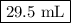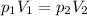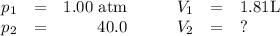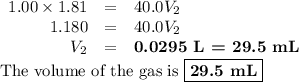Chemistry, 25.07.2019 23:20 chrismeldajbaptiste
Asample of an ideal gas at 1.00 atm1.00 atm and a volume of 1.81 l1.81 l was placed in a weighted balloon and dropped into the ocean. as the sample descended, the water pressure compressed the balloon and reduced its volume. when the pressure had increased to 40.0 atm,40.0 atm, what was the volume of the sample? assume that the temperature was held constant.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1
You know the right answer?
Asample of an ideal gas at 1.00 atm1.00 atm and a volume of 1.81 l1.81 l was placed in a weighted ba...
Questions




Spanish, 24.11.2019 21:31



Mathematics, 24.11.2019 21:31

World Languages, 24.11.2019 21:31





English, 24.11.2019 21:31

Mathematics, 24.11.2019 21:31



Biology, 24.11.2019 21:31