
Chemistry, 25.07.2019 22:20 nataluarenhg6924
The amount of i−3(aq) in a solution can be determined by titration with a solution containing a known concentration of s2o2−3(aq) (thiosulfate ion). the determination is based on the net ionic equation 2s2o2−3(aq)+i3(aq)⟶s4o2−6(aq)+3i−(a q) given that it requires 29.6 ml of 0.260 m na2s2o3(aq) to titrate a 30.0 ml sample of i−3(aq), calculate the molarity of i−3(aq) in the solution.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1


Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3

Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1
You know the right answer?
The amount of i−3(aq) in a solution can be determined by titration with a solution containing a know...
Questions


Mathematics, 07.11.2020 16:40

Mathematics, 07.11.2020 16:40

Mathematics, 07.11.2020 16:40

Physics, 07.11.2020 16:40




Business, 07.11.2020 16:40

Mathematics, 07.11.2020 16:40

English, 07.11.2020 16:40


English, 07.11.2020 16:40



Mathematics, 07.11.2020 16:50

Mathematics, 07.11.2020 16:50

English, 07.11.2020 16:50


 in the solution is, 0.128 M
in the solution is, 0.128 M
 .
.
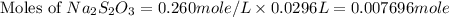
 react with 1 mole of
react with 1 mole of  mole of
mole of 



