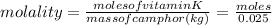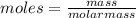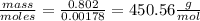Chemistry, 25.07.2019 21:20 Natasha019
Vitamin k is involved in normal blood clotting. when 0.802 g of vitamin k is dissolved in 25.0 g of camphor, the freezing point of the solution is lowered by 2.69 °c. the freezing point and kf constant for camphor can be found here. calculate the molar mass of vitamin k.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 22:30
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1

Chemistry, 23.06.2019 03:30
Select the correct lewis structure for fluorine which is group 7a element?
Answers: 1

Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1
You know the right answer?
Vitamin k is involved in normal blood clotting. when 0.802 g of vitamin k is dissolved in 25.0 g of...
Questions

Mathematics, 01.05.2021 01:00

Social Studies, 01.05.2021 01:00



Mathematics, 01.05.2021 01:00

Mathematics, 01.05.2021 01:00

Mathematics, 01.05.2021 01:00





Mathematics, 01.05.2021 01:00

Social Studies, 01.05.2021 01:00


Mathematics, 01.05.2021 01:00

Mathematics, 01.05.2021 01:00

History, 01.05.2021 01:00



Biology, 01.05.2021 01:00