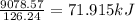Chemistry, 25.07.2019 20:20 NatalieAllen11
Enter your answer in the provided box. pentaborane−9 (b5h9) is a colorless, highly reactive liquid that will burst into flames when exposed to oxygen. the reaction is 2b5h9(l) 12o2(g) → 5b2o3(s) 9h2o(l) calculate the kilojoules of heat released per gram of the compound reacted with oxygen. the standard enthalpy of formations of b5h9(l), b2o3(s), and h2o(l) are 73.2, −1271.94, and −285.83 kj/mol, respectively.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2


You know the right answer?
Enter your answer in the provided box. pentaborane−9 (b5h9) is a colorless, highly reactive liquid t...
Questions

Mathematics, 12.08.2020 06:01

Mathematics, 12.08.2020 06:01









Mathematics, 12.08.2020 06:01









Mathematics, 12.08.2020 06:01

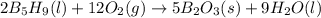
![\Delta H=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0132/3420/76c37.png)
![\Delta H=[(n_{H_2O}\times \Delta H_{H_2O})+(n_{B_2O_3}\times \Delta H_{B_2O_3})]-[(n_{B_5H_9}\times \Delta H_{B_5H_9})+(n_{O_2}\times \Delta H_{O_2})]](/tpl/images/0132/3420/48532.png)
![\Delta H=[(9\times -285.83)+(5\times -1271.94)]-[(2\times 73.2)+(12\times 0)]\\\\\Delta H=-9078.57kJ](/tpl/images/0132/3420/9a745.png)
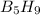 has 63.12 grams of mass
has 63.12 grams of mass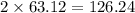 grams of mass
grams of mass