Chemistry, 25.07.2019 18:10 tateandvioletAHS14AY
The combustion of titanium with oxygen produces titanium dioxide: ti (s) + o2 (g) → tio2 (s) when 2.060 g of titanium is combusted in a bomb calorimeter, the temperature of the calorimeter increases from 25.00°c to 91.60°c. in a separate experiment, the heat capacity of the calorimeter is measured to be 9.84 kj/k. the heat of reaction for the combustion of a mole of ti in this calorimeter is kj/mol.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1


Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1
You know the right answer?
The combustion of titanium with oxygen produces titanium dioxide: ti (s) + o2 (g) → tio2 (s) when 2...
Questions

Business, 11.11.2021 14:00

Mathematics, 11.11.2021 14:00


History, 11.11.2021 14:00

Mathematics, 11.11.2021 14:00

Social Studies, 11.11.2021 14:00

Social Studies, 11.11.2021 14:00

Mathematics, 11.11.2021 14:00












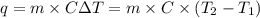
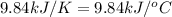
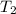 = final temperature =
= final temperature = 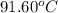
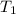 = initial temperature =
= initial temperature = 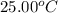
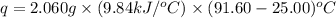
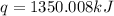
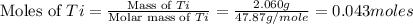
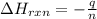
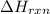 = enthalpy of reaction = ?
= enthalpy of reaction = ?