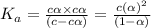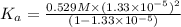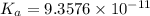Chemistry, 25.07.2019 06:20 swansondonovanp66got
In the laboratory a student measures the percent ionization of a 0.529 m solution of phenol (a weak acid) , c6h5oh, to be 1.33×10-3 %. calculate value of ka from this experimental data.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Plz me ! 1 which of earths spheres contains most of its mass? a atmosphere b hydrosphere c geosphere* d biosphere 2 erosion and weathering are examples of which types of forces? a constructive forces b destructive forces* c gravitational forces d inertia-related forces 3 which of the following statements about earths atmosphere is true? a earths atmosphere contains 78% water vapor which is essentail to life b earths atmosphere contains 21% oxygen c earths atmosphere contains carbon dioxide which all life forms require d earths atmosphere allows radiation from the sun to pass through it and warm earths surface* 4 the strenght of the force of gravity between two objects is determined by which of the following factors? select all that apply a the messes of the objects* b the distance between the objects* c the volumes of the objects d the surface area of the objects 5 earth and moon are kept in there respective orbits due to the influence of a inertia b gravity c gravity and inertia* d neither gravity or inertia if you answer all questions right i will give
Answers: 1

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1

Chemistry, 23.06.2019 06:30
What happens to the glucose molecule during the process of cellular respiration? (5 points) select one: a. it gets broken down. b. it forms oxygen. c. it builds muscles. d. it uses up energy.
Answers: 3

Chemistry, 23.06.2019 11:30
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1
You know the right answer?
In the laboratory a student measures the percent ionization of a 0.529 m solution of phenol (a weak...
Questions


Computers and Technology, 13.03.2020 05:47



History, 13.03.2020 05:47

Computers and Technology, 13.03.2020 05:47






Physics, 13.03.2020 05:48





Mathematics, 13.03.2020 05:48



World Languages, 13.03.2020 05:48

 .
.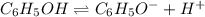
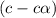
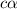
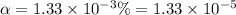
![K_a=\frac{[C_6H_5O^-][H^+]}{[C_6H_5OH]}](/tpl/images/0130/1052/3ceac.png)