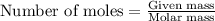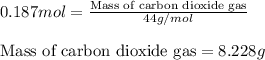Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1

Chemistry, 23.06.2019 11:30
How do you calculate the mass of a product when the amounts of more than one reactant are given?
Answers: 3
You know the right answer?
Determine the mass of co2 produced by burning enough of methane to produce 1.50×102kj of heat. ch4(g...
Questions



Mathematics, 22.04.2020 19:55






History, 22.04.2020 19:55

History, 22.04.2020 19:55


History, 22.04.2020 19:55


Mathematics, 22.04.2020 19:55


Chemistry, 22.04.2020 19:55


Mathematics, 22.04.2020 19:55


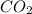 produced will be 8.228 g.
produced will be 8.228 g.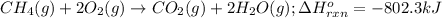
 of carbon dioxide is produced.
of carbon dioxide is produced.