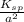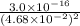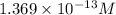Chemistry, 25.07.2019 04:30 wwesuplexcity28
Determine the molar solubility ( ) of zn(cn)2 in a solution with a ph=1.33 . ignore activities. the for zn(cn)2 is 3.0×10−16 . the for hcn is 6.2×10−10 .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1
You know the right answer?
Determine the molar solubility ( ) of zn(cn)2 in a solution with a ph=1.33 . ignore activities. the...
Questions




Mathematics, 25.06.2019 13:50

Mathematics, 25.06.2019 13:50




History, 25.06.2019 13:50


Social Studies, 25.06.2019 13:50



Mathematics, 25.06.2019 13:50

Mathematics, 25.06.2019 13:50



History, 25.06.2019 13:50


Biology, 25.06.2019 13:50

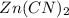 in the given solution.
in the given solution.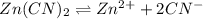
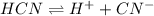
![[H^{+}]](/tpl/images/0129/7944/85507.png)
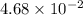
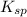 =
= ![[Zn^{2+}][CN^{-}]^{2}](/tpl/images/0129/7944/9ff82.png)
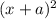
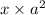 ( x + a = a because a >> x)
( x + a = a because a >> x)