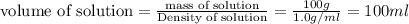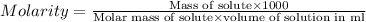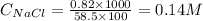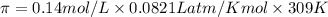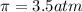Chemistry, 25.07.2019 04:10 connorvoss5805
At 36°c, what is the osmotic pressure of a 0.82% nacl by weight aqueous solution? assume the density of the solution is 1.0 g/ml. (r = 0.0821 l · atm/(k · mol)) a. 7.1 atm b. 0.35 atm c. 0.82 atm d. 4.1 × 102 atm e. 3.5 atm

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3

Chemistry, 23.06.2019 03:30
Mr. rose asked his student to draw a quadrilateral with four unequal sides. an example of this kind of quadrilateral
Answers: 1
You know the right answer?
At 36°c, what is the osmotic pressure of a 0.82% nacl by weight aqueous solution? assume the densit...
Questions

History, 14.07.2019 01:00



Health, 14.07.2019 01:00





History, 14.07.2019 01:00








Business, 14.07.2019 01:00


Mathematics, 14.07.2019 01:00

Chemistry, 14.07.2019 01:00


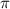 = osmotic pressure = ?
= osmotic pressure = ?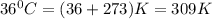
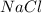 is dissolved in 100 g of solution.
is dissolved in 100 g of solution.