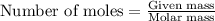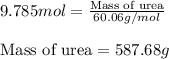Chemistry, 25.07.2019 01:20 1233042260
The emission of no2 by fossil fuel combustion can be prevented by injecting gaseous urea into the combustion mixture. the urea reduces no (which oxidizes in air to form no2) according to the following reaction: 2co(nh2)2(g)+4no(g)+o2(g)→4n2(g)+2c o2(g)+4h2o(g) suppose that the exhaust stream of an automobile has a flow rate of 2.32 l/s at 670 k and contains a partial pressure of no of 12.1 torr. what total mass (in gram) of urea is necessary to react completely with the no formed during 8.1 hours of driving?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
How much energy moves onto the next level, in an energy pyramid
Answers: 1

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 3

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

You know the right answer?
The emission of no2 by fossil fuel combustion can be prevented by injecting gaseous urea into the co...
Questions




Mathematics, 08.05.2021 02:40


Mathematics, 08.05.2021 02:40



History, 08.05.2021 02:40

Mathematics, 08.05.2021 02:40


Mathematics, 08.05.2021 02:40



Mathematics, 08.05.2021 02:40


Mathematics, 08.05.2021 02:40

Social Studies, 08.05.2021 02:40


Mathematics, 08.05.2021 02:40

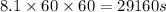
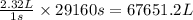
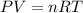
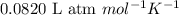
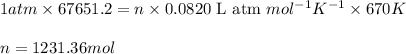
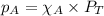
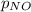 = partial pressure of NO = 12.1 torr
= partial pressure of NO = 12.1 torr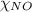 = mole fraction of NO = ?
= mole fraction of NO = ?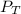 = total pressure of solution = 1 atm = 760 torr (Conversion factor: 1 atm = 760 torr)
= total pressure of solution = 1 atm = 760 torr (Conversion factor: 1 atm = 760 torr)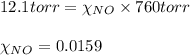
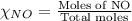
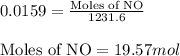
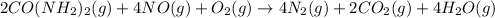
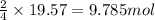 of urea.
of urea.