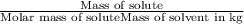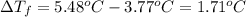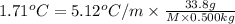Chemistry, 25.07.2019 00:30 allstar976
When a 33.8-g sample of an unknown compound is dissolved in 500. g of benzene, the freezing point of the resulting solution is 3.77°c. the freezing point of pure benzene is 5.48°c, and kf for benzene is 5.12°c/m. calculate the molar mass of the unknown compound.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
The density of a planet is 0.69 g/cm3 (density of water is 1.0 g/cm3). which of the following planets might this be? a. mercury b. venus c. saturn d. mars
Answers: 3

Chemistry, 21.06.2019 20:40
Astudent made the lewis dot diagram of a compound shown. what is the error in the lewis dot diagram? a)an o atom should transfer all of its six electrons to mg because the formula is mgo b) both electrons of mg should be transferred to one o adam because the formula is mgo c) the electrons should be transferred from each o add him to capital mg because mg has fewer electrons d) the number of dots around mg should be four because it has to transfer two electrons to each o
Answers: 1

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1
You know the right answer?
When a 33.8-g sample of an unknown compound is dissolved in 500. g of benzene, the freezing point of...
Questions



History, 05.05.2020 12:35


Mathematics, 05.05.2020 12:35

Arts, 05.05.2020 12:35

Mathematics, 05.05.2020 12:35

Biology, 05.05.2020 12:35


English, 05.05.2020 12:35


History, 05.05.2020 12:35


Mathematics, 05.05.2020 12:35

Mathematics, 05.05.2020 12:35




English, 05.05.2020 12:35

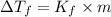
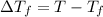
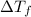 = change in boiling point = 0.81 K
= change in boiling point = 0.81 K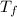 =Boiling point of the solution = 3.77°C
=Boiling point of the solution = 3.77°C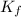 = freezing point constant = 5.12°C/m
= freezing point constant = 5.12°C/m