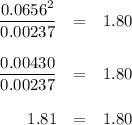Phosphorus pentachloride decomposes according to the chemical equation pcl5(g)↽−−⇀pcl3(g)+cl2(=1.80 at 250 ∘c a 0.197 mol sample of pcl5(g) is injected into an empty 2.90 l reaction vessel held at 250 ∘c. calculate the concentrations of pcl5(g) and pcl3(g) at equilibrium.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3

Chemistry, 23.06.2019 04:00
Why must humans find substitutes for many minerals found on earth? (a) form at an extremely slow rate (b) controlled by other countries (c) too deep in the earth to collect
Answers: 1
You know the right answer?
Phosphorus pentachloride decomposes according to the chemical equation pcl5(g)↽−−⇀pcl3(g)+cl2(=1.80...
Questions


World Languages, 24.07.2019 16:00


English, 24.07.2019 16:00

Biology, 24.07.2019 16:00


Mathematics, 24.07.2019 16:00




Mathematics, 24.07.2019 16:00

English, 24.07.2019 16:00


Mathematics, 24.07.2019 16:00

Mathematics, 24.07.2019 16:00


Mathematics, 24.07.2019 16:00


Mathematics, 24.07.2019 16:00

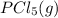 is 0.0655 M and
is 0.0655 M and 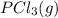 is 0.00240 M at equilibrium.
is 0.00240 M at equilibrium.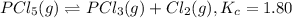
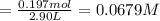

![[PCl_3]=x](/tpl/images/0128/9812/3c44a.png)
![[Cl_2] = x](/tpl/images/0128/9812/a7a23.png)
![=[PCl_5]= (0.0697- x)](/tpl/images/0128/9812/ead0f.png)
![K_c=\frac{[PCl_3][Cl_2]}{[PCl_5]}\\\\1.80=\frac{x\times x}{(0.0679-x)}\\\\x = 0.0655](/tpl/images/0128/9812/79ef0.png)
![=[PCl_5]= (0.0679- x) = (0.0679 -0.0655 )M=0.00240 M](/tpl/images/0128/9812/ac2f8.png)
![= [Cl_2] = x = 0.0655 M](/tpl/images/0128/9812/6faf5.png)
![\text{[PCl$_{5}$]} = \dfrac{\text{0.197 mol}}{\text{2.90 L}} = \text{0.067 93 mol/L}\\\\](/tpl/images/0128/9812/66356.png)
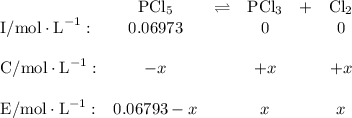
![K_{\text{c}} = \dfrac{\text{[PCl$_3$][Cl$_2$]}}{\text{[PCl$_5$]}} = \dfrac{x^{2}}{0.06793-x} = 1.80\\\\\begin{array}{rcl}\\x^{2}& = & 1.80(0.06793 - x)\\x^{2& = & 0.1223 - 1.80x\\x^{2} + 1.80x - 0.1223& = & 0\\x & = & \mathbf{0.0656}\\\end{array}](/tpl/images/0128/9812/cf1bb.png)